The research interest of the Electrochemistry and Corrosion Laboratory range from the preparation of advanced functional materials for energy, environment and biomedical, especially, from natural resources and solid waste using various methods such as electrochemical, electrospinning, and electro spray. Examples of advanced functional materials we are developing including electrocatalyst for oxygen reduction reaction, catalyst for esterification, high-capacity liquid absorbents, etc. Below are some projects we are currently conducted.
Current Projects
Integrated capture and conversion systems for CO2 electroreduction to valuable chemicals
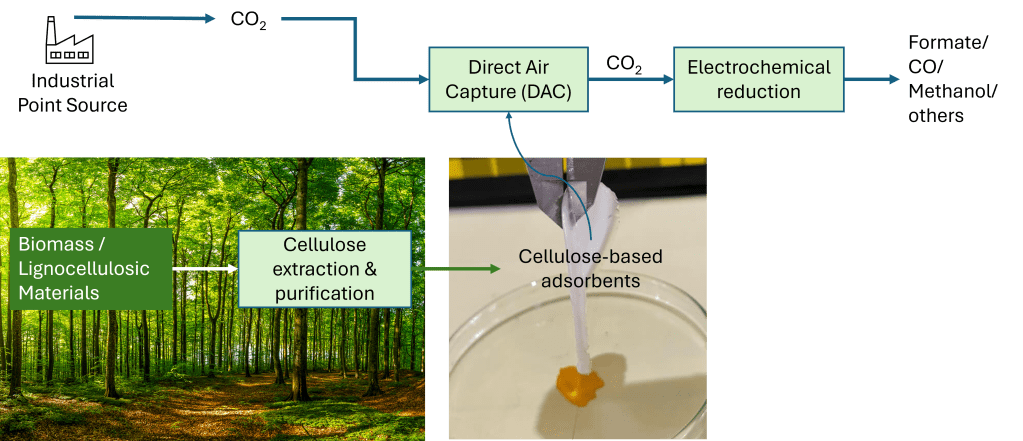
The goal of this project is to develop an integrated system to transform CO2 into valuable chemical products such as methanol, ethylene, nitrogen fertilizer, and so on. The schematic diagram describes one examples of the system proposed in palm oil industry. Our works concern with the development of electrochemical reduction of carbon dioxide process (electrocatalysts, reactor system, and related issues) and of designing cellulose-based adsorbents from empty fruit bunches. The electrocatalysts developed are for both CO2 electroreduction and its counter electrode oxygen evolution reaction. They will be applied in a full electrochemical reactor system. Design and scale up of such kind of reactor are also investigated. This project is supported by Indonesia-NTU Singapore Institute of Research for Sustainability and Innovation (INSPIRASI) and PT. Pertamina Persero.
Development of metal-air batteries as green and safe energy sources
The goal of this project is to develop metal air-battery system for either electrical vehicles or stationary applications. Self-corrosion and passivation of metal anodes and the sluggish oxygen reduction reaction (ORR) in cathodes represent a bottleneck for the development of such technology. The working principle of the metal-air battery is as follows (see figure). The electrolyte can be basic (e.g., KOH) or neutral (e.g., NaCl) solutions that separates the anode and cathode to facilitate the transfer of ion carrying the electrical charge. Anode is a metal (e.g., Mg, Al, Zn, Li) that can easily release electron through oxidation reaction. Cathode is carbon material coated with an electrocatalyst for ORR to change oxygen into hydroxyl ions. When circuit is closed, electrical is drawn, metal dissolves to release electron. Metal ion is transported to cathode via electrolyte. At anode, metal reacts with electrolyte to form hydrogen, which is undesirable side reaction.
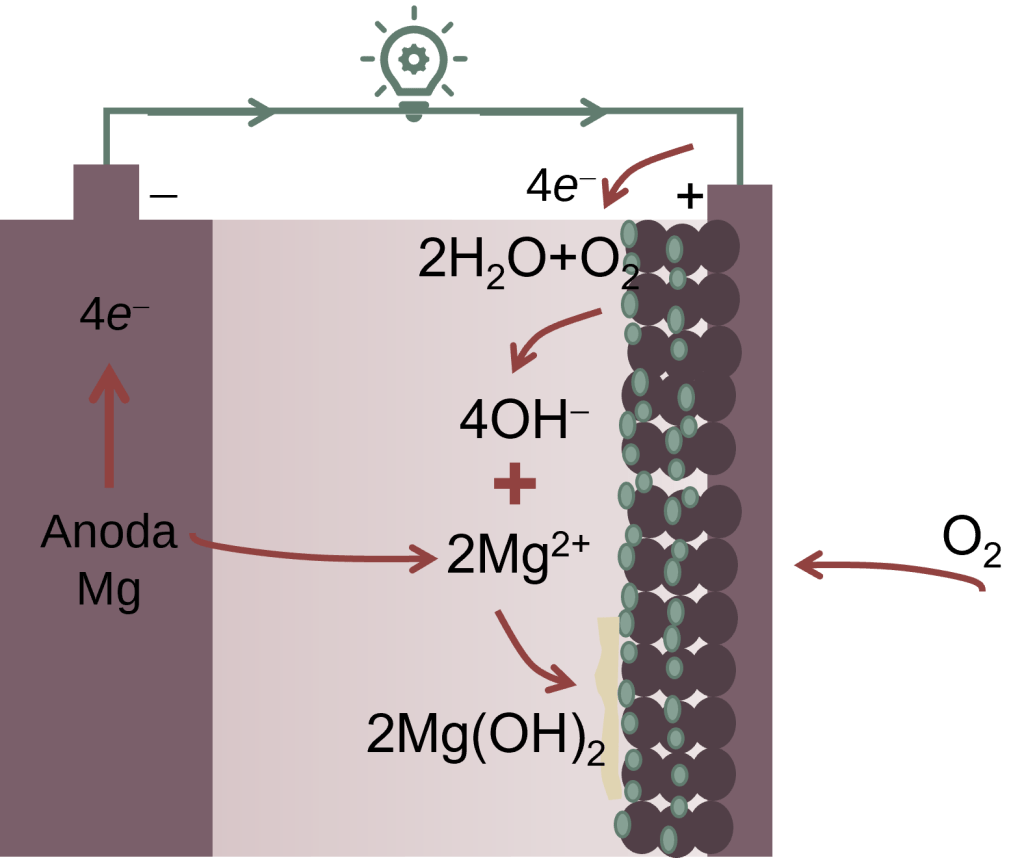
The electrocatalysts we develop include magnetite (Fe3O4), mangan dioxide (MnO2) and N-doped carbon aerogel derived from biomass such as coir fiber and empty bunches of palm. Some of the results have been published in reputable scientific SCI journals such as Chemical Engineering Science, Journal of Nanoparticle Research, Advanced Powder Technology, etc. (see here). Further works are currently still in progress to optimize its performance.
Adv. Powder Technol. (2020) Chem. Eng. Sci. 201,112 (2019) J. Cer. Soc. Japan 126, 906 (2018) J. Powder Technol. Adv. Func. Mat. 1, 1 (2018) J. Chem. Eng. Japan 49, 144 (2016) Asia-Pac. J. Chem. Eng. 9, 768 (2014) Adv. Powder Technol. 24, 507 (2013) J. Nanopart. Res. 14, 807 (2012) Adv. Powder Technol. 23, 328 (2012) KONA Powder Part. J. 36, 145 (2019)
Development of solar steam generation (SSG) for fresh water supply from sea water
The goal of this project is to develop a system that can produce fresh water from sea water. The system consists of a photothermal material that absorbs the incoming light and convert it into heat and a substrate that can localize heat in the evaporation surface, minimize heat and has capability to transport water to the surface through its pores. The photothermal materials we are developing include magnetite nanoparticles, lignin nanoparticles and their composite. Lignin nanoparticles are derived from biomass, e.g., coir fiber. Biomass is also used as raw materials to develop the substrate in the form of cellulose aerogel. This is an effort to minimize, or even, to eliminate waste because both the waste of delignification process to produce pulp as the raw material for cellulose aerogel is recovered and processed further to become lignin nanoparticles that serve as photothermal material in SSG system.

Some of the results have been published in reputable SCI journals such as Colloid and Surfaces A, Microporous and Mesoporous Materials, Advanced Powder Technology, etc. (see here). Further works are still undergoing to optimize the system.
Cellulose 26, 9583 (2019) Adv. Powder Technol. 31, 1412 (2020) Adv. Powder Technol. 31, 3267 (2020) Cogent Eng., 7, 1748962 (2020) BCREC, 5 538 (2020) Micropor. Mesopor. Mat. 218, 95 (2015) Coll. Surf. A 476, 1 (2015) Adv. Powder Technol. 25, 1593 (2014) J. Non-Cryst. Solid 400, 6 (2014) Asia-Pac. J. Chem. Eng. 7, 448 (2012) Adv. Powder Technol. 20, 468 (2009)
Development of hydrophobic solid acid catalyst for esterification
The goal of this project is to develop a hydrophobic solid acid catalyst for specifically esterification reaction that produces water as byproduct. We have successfully developed a process to fabricate cellulose/carbon-based materials from natural resources for use as sorbent materials or other applications such as heat insulator, solar thermal conversion materials, electrocatalyst, catalyst, etc. We have devised a cheap and green method to convert coir fiber into compressible and ultralight cellulose aerogel using alkali-urea method and freeze drying. The cellulose aerogel derived from the coir fiber, an abundantly available agricultural waste materials, has high absorption capacity towards water, oil and dyestuffs. The aerogel has a macroporous structure, ultralight density, high porosity, good durability, and thermal stability. Carbonizing the cellulose aerogel under inert environment at high temperature produce carbon aerogel that inherit the microporous structure of cellulose aerogel.
The works have been published in reputable SCI journals such as cellulose (see here). Further works are still undergoing to optimize the process and to fit the aerogel for specific applications.
Cellulose 26, 9583 (2019) | Adv. Powder Technol. 31, 1412 (2020) | Adv. Powder Technol. 31, 3267 (2020) | Cogent Eng., 7, 1748962 (2020) | BCREC, 5 538 (2020)
Funding & Collaborations
The works are funded by the Ministry of Education and Culture, the Ministry of research and Technology, and Japan Science and Cooperation Agency (JICA) through JICA-Predict2, LPDP and grant-in-aids from ITS. We also receive support from some companies, e.g., PT. Pertamina (Persero), PT. PLN, PT. Trans Jawa Sulawesi, PT. Petrokimia Gresik for providing urea and steel plate, and PT. PQ Silica Indonesia for water glass. Some research projects are in collaboration with other universities including Hiroshima University (Prof. Takashi Ogi), Tokyo University of Agriculture and Technnology (Prof. Wuled Lenggoro), National Taiwan University of Technology (Assoc. Prof. Min-Hsin Yeh), Nanyang Technological University (Assoc. Prof. Lydia Wong), Universitas Sebelas Maret (Prof. Agus Purwanto), and Universitas Padjadjaran (Prof. I Made Joni, Prof. Camellia Panatarani).

